HA vs. Ti Implant Long-Term Success Rate and Causes of Failure
-Investigation on Potential of HA-coating-
Introduction
Numerous reports casting doubts on the long-term stability and prognosis of hydroxyapatite (HA)-coated implants have been published [1]. These reports point out that unstable HA coating elevates the sensitivity to bacterial infection, possibly leading to rapid bone breakdown or saucerization bone defect and that HA-coated implants have no features superior over titanium (Ti) implants [2]. However, the majority of these reports were anecdotal in nature, relying on the data from isolated case report [3]. These reports began to be published early in the 1990s, 5 years after 1984 when the clinical application of HA-coated implants was started, and they attributed the failure of this type of implant to the lack of long-term stability of the coating layer. The present study was undertaken to review and verify these previous reports from 3 points of view: (I) comparison between .ndings from statistical analysis of HA-coated thread type implants (implants kept placed for 5 years or longer among the 1157 HA-coated thread type implants bearing loads for 6 months longer; a type of implant adopted at multiple centers after 1995) and the .ndings from statistical analysis of Ti implants; (II) classi.cation of the bone defect patterns in relation to clinical symptoms in cases of implant failure among the subjects of this study and comparison with Ti implants; and (III) evaluation of long-term advantages and risks based on the overall assessment of histological features revealed by topography.
I.Report of the study
HA-coated implants are expected to enhanced osteointegration
and appear to be useful, particularly in sites with poor bone quantity
or quality. Initial success in the use of HA-coated implants resulted in
increased frequency of their clinical use. However, despite their
clinical application since 1984, only a small number of reports have
been published on HA-coated implants. Further, a number of reports
doubting the long-term stability and prognosis have been published.
The most strongly criticized feature of clinical use of HA-coated
implants is the lack of statistical reports endorsing the long-term
stability of HA-coated implants. When conducting a survey on the
long-term course of HA-coated implants, the following are important.
The survey involves multiple implants.
At least 5 years have elapsed after prosthetic treatment for each
subject surveyed.
The number of implants lost before prosthetic treatment was
excluded from analysis.
Data from subjects on whom con.rmation is not possible by means
of recall, etc., are excluded from analysis.
Criteria for success rate are prepared in advance, including factors
such as mobility and bone resorption rate.

Materials and Methods
Two types of .xtures with different surface properties were employed for this study. (Phisio Odontram Implant (POI) System, Osaka, Japan). One of them was a POI System Finafix made of titanium alloy Ti-6Al-4V (ELI). It is a titanium thread type implant with a surface roughness of 2.7 um and a 135140 nm anode-oxidized layer. The other was a POI System Finatite, which is an HA-coated thread type implant having a 20 µm thick HA coating layer applied by .ame spraying (3000°C) onto the 1 mm oxidized membrane. Its Ca/P ratio is 1.66 (Ca/P of bone = 1.67). The crystallization rate is 55%, and the coating layer is located beneath the mirrorpolished layer and the 2.7 blast layer (Fig. 1). The criteria for the evaluation of success rate of implants were prepared by adding our original elements to the 1988 Toronto Consensus Criteria. During the 13-year period from 1995 to 2008, 1157 pieces of HA implant were placed. Of these implants, 772 remained placed. For less than 5 years and 385 remained placed for 5 years or more. There were 128 patients with a mean age of 55 (SD = 10.1). The present study covered implants that could be followed for 5 years or more after prosthetic treatment. Six implants failed before prosthetic treatment. Hence, prosthetic treatment was performed on 379 implants. Of these implants, 57.1% were placed into the upper jaw and 42.9% into the lower jaw. The prosthetic design was most frequently the single crown, followed by ?xed partial Br and full-arch Br. Removable prosthetic appliance was used rarely. Of the patients who did not participate in a follow-up appointment for the study sample, 16 showed no interest in follow-up, 3 were deceased, and 8 were unable to contact by moving out and changing clinics. Of the 128 patients who received the implants, 101 patients carrying 333 implants were included in subsequent statistical analysis. The data were analyzed statistically by Wilcoxon test using the computer program SAS9.1 (SAS Institute, Cary, NC), with 430 Ti implant (inserted during the same period) serving as the control group (Figs. 2 and 3).
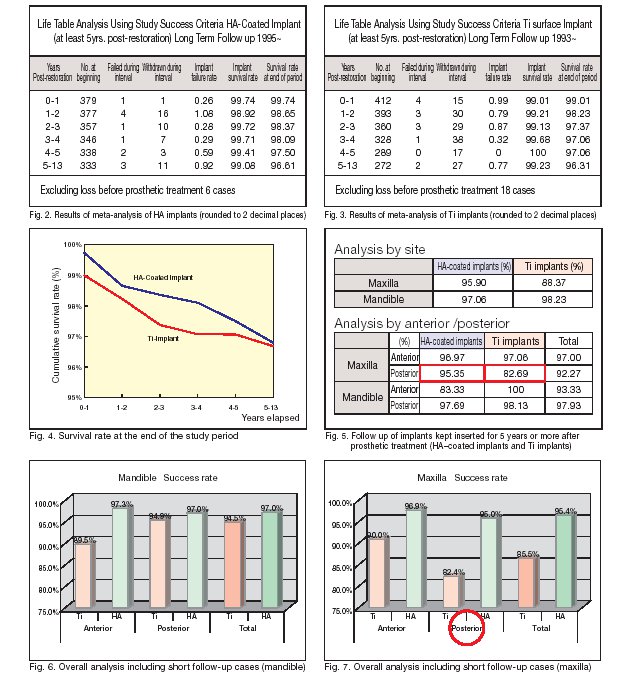
Results of statistical analysis
Fig. 2 shows the results of meta-analysis of HA-coated implants. As described above, the number of implants that failed before loading was 6. When data were processed at the end of each subsequent year, the number of failed implants ranged from 1 to 4 per year, and 1?16 withdraws (drops out on research) were observed per year. The cumulative success rate for cases elapsing 5?13 years after the beginning of loading was 96.61%. A noteworthy ?nding from the meta-analysis of Ti implants (Fig. 3) is that 18 implants had failed before loading. If this result is combined with the fact that the level of technical error was identical to that of HA-coated implants, it seems likely that healing immediately after insertion and initial Ti implants were processed at the end of each subsequent year, revealing that the number of failed implants was 0?4 per year, 15?38 withdraws(drops out on research) were observed per year and the cumulative success rate for the cases elapsing 5?13 years after the beginning of loading was 96.31%. Fig. 4 shows a graph comparing the survival rate at the end of the follow-up period between Ti implants and HA-coated implants. Both groups depicted a similar downward curve while maintaining the difference in failure rate observed soon after prosthetic treatment. There was no signi?cant difference in the results between the 2 groups at a signi?cance level of 0.05. A noteworthy ?nding is that when the results were analyzed by site, a signi?cant difference was noted in the upper molar implants between the 2 groups (Figs. 5 through 7).
Summarized results of statistical analysis
1) In the follow-up study of 385 HA-coated implants for 13 years, the success rate for 513 years was 96.61%. When analyzed for maxilla and mandible separately, the success rate was 97.06% for mandible and 95.90% for maxilla. 2) With regard to the early outcome of HA-coated implants, Wheeler [4] reported that failure began to appear several years after prosthetic treatment and that many implants failed thereafter, accompanied by peri-implantitis. In the present study, however, the success rate of HA-coated implant decreased from 98.09% (4 years after prosthetic treatment) to 97.50% (5 years after treatment), but this change during the one-year period was not statistically signi.cant (Fig. 2), and no case followed the clinical course of failure similar to the one described above.
3) The long-term success rate did not differ signi.cantly between HA-coated implants (96.61%) and Ti implants (96.31%). A noteworthy .nding from this long-term comparison was a site-speci.c signi.cant difference, i.e., signi.cant difference in upper molar implant success rate between HA-coated implants (95.35%) and Ti implants (82.69%).
II.Classi.cation of bone defect patterns around the failed implants
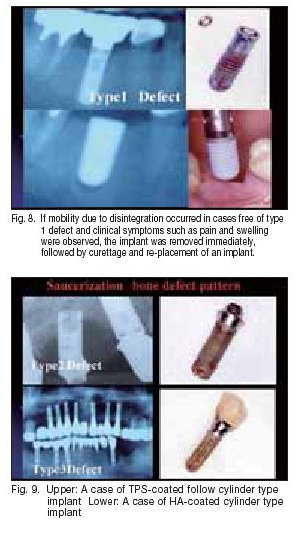
Fig. 9. Upper: A case of TPS-coated follow cylinder type The data collected on implant failure in this study were analyzed in more detail, and the failure was divided into 3 patterns depending on radiological and clinical features: Type 1 defect (horizontal and vertical defect on X-ray is below 1 mm; symptoms such as pain and infection not observed; implant withdrawal and immediate re-placement possible), Type 2 defect (horizontal and vertical defect on X-ray over 1 mm; symptoms such as acute bone destruction and infection/pain observed rarely; implant withdrawal and immediate re-placement possible if infection is absent and initial ?xation is achieved), and Type 3 defect (bone defect beyond root apex visible on X-ray, accompanied by fenestration and cleavage; often presenting symptoms such as acute bone destruction and infection/pain; re-placement immediately after withdrawal impossible).
Comparison of failure patterns
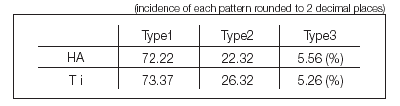
As shown in the table above, there was no signi?cant difference in the bone defect patterns between HA-coated implants and Ti implants. During routine clinical care, Type 1 defect, showing mobility due to disintegration, was dealt with by the removal of the ?xture and surrounding tissue debridement, followed by immediate placement of a new slightly larger diameter ?xture (Fig. 8). In Type 2 defect cases, implant withdrawal and immediate re-placement were possible if infection was absent and initial ?xation was achieved. Type 3 defect is the severest bone defect, often accompanied by symptoms such as infection and pain, and we judged it impossible to perform implant withdrawal and immediate re-insertion in such cases (Fig. 9).
III.Materials and methods for histological evaluation
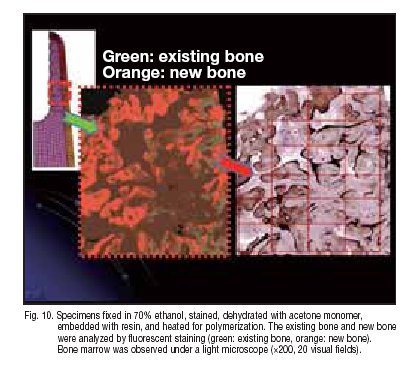
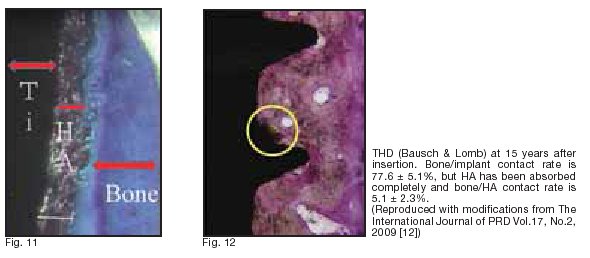
An adult female with single crown prosthetic HA-coated implant having elapsed 2 years after loading. Because of Abutment screw trouble, the implant was removed with Trepine Bur. After obtaining the patientes consent, the removed implant was embedded and ?xed for histological examination of the longitudinal and transverse sections. Like the method of processing bone biopsy samples, the removed implant was subjected to 70% ethanol ?xation, staining, acetone monomer dehydration, resin embedding, and heating for polymerization. Bone and surrounding tissue were observed by staining with toluidine blue, and the tissue structure was observed staining with toluidine blue, and the tissue structure was observed under an electron microscope. This was followed by evaluation of 20 visual ?elds with ?uorescent staining to determine the BIC rate (bone-implant contact rate) on the longitudinal section (Fig. 10).
Results of histological evaluation
Around the titanium alloy, a 20 Êm HA-coating layer and the surrounding 100 Êm bone-like tissue were observed. When observed under light and electron microscopes (~20?300), the connection of HA coating to mature bone was visible (Fig. 11). No void or ?brous tissue was observed on the implant-bone interface, and no aberrant epithelial tissue or in?ammatory cell in?ltration was detected. No foreign body reaction was observed around the implant. At some sites, direct binding of osteoblasts to HA was noted. Then, on the longitudinal section, the existing bone and new bone were examined with ?uorescent staining to calculate the BIC rate 5.1 } 2.3%. (green: existing bone, orange: new bone). Measurement was performed for 20 visual ?elds under a light microscope (~200). BIC rate was approximately 60%. The HA-coating layer had been absorbed slightly more markedly in the direction along the crown.
Discussion
Around the titanium alloy, a 20 Êm HA-coating layer and the surrounding 100 Êm bone-like tissue were observed. When observed under light and electron microscopes (~20?300), the connection of HA coating to mature bone was visible (Fig. 11). No void or ?brous tissue was observed on the implant-bone interface, and no aberrant epithelial tissue or in?ammatory cell in?ltration was detected. No foreign body reaction was observed around the implant. At some sites, direct binding of osteoblasts to HA was noted. Then, on the longitudinal section, the existing bone and new bone were examined with ?uorescent staining to calculate the BIC rate 5.1 } 2.3%. (green: existing bone, orange: new bone). Measurement was performed for 20 visual ?elds under a light microscope (~200). BIC rate was approximately 60%. The HA-coating layer had been absorbed slightly more markedly in the direction along the crown.
HA coating has clinical advantages (promotion of integration and effectiveness on sites with poor bone quantity or quality). However, long-term stability of HA coating has been considered doubtful. Indeed, HA coating is susceptible to the in?uence of bio?lm, and the methods of HA coating involving a high risk (possibly affecting long-term stability) have been used in the past (Fig. 9). Clinicians should seriously review these past problems. However, despite such concerns with HA coating, the long-term failure rate for HA-coated implants that remain inserted for 5 years or more after prosthetic
treatment had not increased markedly, suggesting that HA coating is
unlikely to serve as a factor responsible for the failure of implants in
the long-term follow-up surveys.
Attempts of enhancing bone binding to implant surface can be
roughly divided into the coating method (HA, TPS, Sintered, Oxides)
and the un-coating method (SLA, Osseotite, TiUnite). As compared
to ?rst-generation implants, these second-generation implants have
an overwhelmingly higher potential of stimulating the binding of
osteoblasts and making the implant stronger. Furthermore, the
materials used for second-generation implants are superior also in
terms of surface adhesiveness (due to the coarse surface.
Furthermore, second-generation implants are higher in terms of
cell-differentiating potential in vitro as well as in terms of contact rate
with surrounding bone, binding power, and ?xative power in vivo
[5?10]. Buser et al. [11] veri?ed the relationship between implants
with coarse surface and the implant-bone contact rate in 1991.
According to their report, the implant-bone contact rate was
20%?25% for implants with sandblast and acid pickled surface,
30%?40% for implants with TPS coating (sand-blast large grit and acid-etched and titanium plasma-sprayed), 50%?60% for SLA (sand-blast large grit and acid textured), and 60%?70% for HA-coated implants. Many other reports providing similar results have been published. Recently, a histological study was reported, demonstrating that an HA-coated ?xture removed after a long time (15 years) after insertion showed almost complete absorption of the HA coating layer and noninvasive direct contact between Ti surface and bone as a result of long-term repeated remodeling [12] (Fig. 12).
Some investigators reported that the infected HA-coated ?xture is
destroyed by the surrounding tissue [8], while other investigators
reported that Haversian canal was observed in the vicinity of implant
surface and that the normal bone remodeling correlated with HA
absorption [12]. In the latter report, the HA isolated from the
HA-coated ?xture showed no sign of foreign-body reaction, and it
was shown that ossi?cation occurred in the HA-absorbed area,
similar to the ?nding reported by Hardy and Frayssinet [13].
To date, however, very few reports have been available concerning
the relationship with soft tissue. In this connection, Block et al. [14]
published a noteworthy report, in which he suggested that when
HA-coated mandibular implants were followed for 10 years, the
failure rate was only 2.9% for patients having keratinized gingiva, but
as high as 29.5% for patients free of keratinized gingiva, accompanied by poor cleaning status in the latter group. A skill used to avoid the exposure of implantfs HA-coating layer into the oral cavity is to
arrange the polished plane (called gcrest moduleh) or the un-coating
later on the side of the coating layer facing the crown. In addition,
there is a report demonstrating that the HA coating layer can
adequately resist changes in pH and remains stable even when it is
exposed into the oral cavity. The implant body has a macroscopic
design, whereas the crest module is often smoother to impair plaque
retention if crestal bone loss ocure. The apical dimension of the crest
module varies greatly from one system to another@(0,5mm to
5mm).
Because special environments (mucosa-perforating area) are
involved during dental management, minute processing of this part
by un-coating to elevate the potential of integration will work
favorably. It is desirable to introduce the alkali heating technique
(clinically introduced in the ?eld of hip-joint management: AHFIXR)
[15], outcome of technological innovation at the molecular level such
as nano-size HA particle coated surface (NanoTiteR) and macrodesigns
(platform switch, etc.) facilitating the stabilization of the quality and quantity of this area and resistance to bone resorption during loading. As shown in the analysis conducted during this study, HA coating
of the surface of the implant within the bone can lead to high success
rate in the upper posterior region and allow the acceleration of
integration and consolidation of the implant inserted into relatively
soft bone. This coating also appears to be bene?cial when the
implant is placed into the socket after tooth extraction or the regenerated bone (sinus lift, etc).
Assuming that the cause for the failure of HA-coated implants was
similar to that for the failure of Ti implant in the cases covered in this
study, how does the failure begin? Sauce-shaped early bone resorption at the neck of implant can cause a condition akin to that observed in the periodontal pocket. Regardless of the shape of
implants, many reports from statistical analysis revealed that the
amount of bone resorption at the tooth neck occurred rapidly during
the ?rst year [16?19]. According to the measurement performed with
reference to the ?rst screw thread by Adell et al. [17], bone resorption
at the bone apex was large, particularly during the ?rst year [mean NEWS
1.5 (3.3) mm], and the resorption in subsequent years was smaller
(0.05?0.13 mm/year). Misch [20] studied the cause for early bone
resorption at the bone apex, citing the hypotheses given below.
@ Periosteal re?ection hypothesis
A Implant osteotomy hypothesis
B Autoimmune response of host hypothesis (associated with bacteria)
C Biological width hypothesis
D Mechanical stress factors hypothesis
Misch reported that hypotheses through cannot explain the
cause of resorption. Hypothesis is valid to some extent but cannot
fully explain the cause. He supported the mechanical element
most strongly.
Indeed, bone can change in response to stress. Frost [21] divided
the osseous tissue associated with mechanical adaptation to
pre-fracture strain force into the following 4 window: (a)Acute disuse
atrophy window, (b) Adapted window, (c) Mild overloading window
(stimulating calci?cation), and (d) pathologic overload (fatigue fracture
and bone resorption). Furthermore, the changes of bone in response
to stress can vary depending on the maturity level, hardness, and the
amount of bone exposed to stress; further, it appears that the bone
around the implant is exposed to risk during the ?rst year after
prosthetic treatment and that the risk becomes lower in the second
and subsequent years because of further bone maturation and
stabilization of bone hardness and amount.
According to the recent mechanical studies on dental implants, the
resistance of bone is the highest to compressive force (}0%) and
lower to tensile force (?30%) and shear stress (?65%). With many
implants, the shear stress arising from occlusion is converted at the
?rst screw thread into compressive force or tensile force, and bone
resorption is prevented by 40%?70% elevation in resistance to such
forces. Even a slight (0.25 mm) increase in implant diameter leads to
as much as 5%?10% increase in surface area. Therefore, when
mechanical elements are taken into account during clinical planning,
the implant diameter is more important than the implant length.
Wonejae Yu et al. [22] conducted a mechanical study of the stress
loading area at varying implant diameters and bone apex widths,
using the ?nite element method. In that study, a saucer-shaped
stress loaded area was observed at the bone apex corresponding to
the implant neck, and it was quite akin to the form of initial bone
resorption. Wide-body implants with a larger diameter are mechanically
more useful than elongated standard body implants. However,
they involve a risk for reducing the bone width (biological width).
Tissue with both small width and not supported by bone marrow is
likely to fail during the acute or subacute stages. Among others,
cortical bone lacking marrow cavity is poor in regenerative potentials
and is likely to be resorbed. Furthermore, since biological width
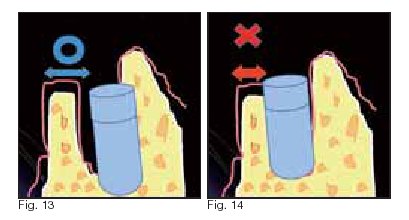
encompasses a horizontal dimension as well, the author thinks that
bone tissue possessing marrow cavity with a regenerative potential
needs to have at least 2 mm thickness of bone/periosteum (Fig. 13
and 14).
Assuming that the cause for the failure of HA-coated implants is
identical to that for the failure of Ti implants, the failures observed
during this study may be attributed to the concentration of stress on
the premature bone or thin cortical bone at the apex facing the
implant neck, resulting in the beginning of bone resorption and
creation of a condition akin to periodontal pocket, and excluding the
failures attributable to pre-loading factors (surgery, ?xture surface
properties, patientfs factors). If this view is valid, the implant diameter
and the design of its neck will be important. Wide-body implants
should be inserted into a location within the existing tissue where
post-healing bone resorption and adequate width of regeneration
(biological width) are assured. If such a biological width is absent,
early bone resorption may occur, possibly leading to the failure of the
implant.
Conclusion
The results of the 13-year evaluation of second-generation thread type HA-coated implants in the present study were clinically satisfactory. A noteworthy .nding from this long-term comparison was a site-speci.c signi.cant difference, i.e., signi.cant difference in upper anterior implant success rate between HA-coated implants and Ti implants. The cause of failure, as analyzed from the patterns of radiological bone defects and clinical symptoms, appears to differ little between these implants and Ti implants. Some requirements revealed in this study seem to be useful in elevating the predictability of integration. Acknowledgments: The author is indebted to Professor Kanichi Nakagawa (Tokyo Dental College) and Professor Ichiro Nishimura (UCLA) for their advice about basic and clinical implantology for a long period of time. Part of this paper was presented at the 38th meeting of the Japanese Society of Oral Implantology (2008), The First ICOI Japan Advanced implant symposium (2009), and the JMM Colloquium (2009).
References
1)BIESBROCK, A.R.and EDGERTON, M. :Evaluation of the clinical predictability of hydroxyapatite-coated endosseous dental implants: A review of the literature ;Int.
J ,Oral Maxillofac. implants,10:712-720,1995.
2)ALBREKTSSON, T.and SENNERBY, L. :State of the art in oral implants ;J. Clin.
Periodontal,18:474-481,1991.
3)LOZADA, J.L.and JAMES, R.A.and BOSKOVIC, M.: HA-coated implants.
Warranted or not? ;Compend Contin Educ. Dent., 15(suppl):539-543,1993.
4)WHEELER, S.,L. :Eight-year clinical retrospectve study of titanium plasma-sprayed
and hydroxyapatite-coated cylinder implants ; Int .J. Oral Maxillofac. Implants,
11:340-350,1996.
5)MASUDA T, YLIHEIKKILA PK, FELTON DA, Cooper LFF. Generalizations regarding
the process and phenomenon of osseointegration. Part. In vivo studies. ; Int .J.
Oral Maxillofac. Implants ,13:17-29,1998.
6)TAKEUCHI K, SARUWATARI L, NAKAMURA HK, YANG JM, OGAWA TF.
Enhancedintrinsic biomechanical properties of osteoblastic mineralized tissue on
roughened titanium surfaceGJ. Biomed Master Res A, 72A:296-305 ,2005.
7)BUTZ F,AITA H, TAKEUCHI K, OGAWA T. : Enhanced mineralized tissue adhesion
to titanium over polystyrene assessed by the nanoscratch test. ; J. Biomed Mater
Res A, 74: 164-170, 2005.
8)BUTZ FCAITA HCWANG CJCOGAWA T. FHarder and stiffer bone osseointegrated to
roughened itaniumG J. Dent Res ; 85: 560-565, 2006.
9)OGAWA T, NISHIMURA I. : Different bone integration profiles of turned and
acidetched implants associated with modulated expression of extracellular matrix
genesG. Int J. Oral Maxillofac Implants, 18:200-210, 2003.
10)OGAWA T, OZAWA S, SHIN JH, RYU KH, SUKOTJO C, YANG JM, et al. :
Biomechanical evaluation of osseous implants having different surface topographies
in ratsG. J. Dent Res, 79:1857-1863, 2000.
11)BUSER, D., SCHENK, RK., STEIMEMANN, S., FIORELLINI, JP.,FOX, CH. and
STICH, H. : Influence of surface characteristics on bone integration of titanium
implants. A histomorphometric study in miniature pigs ;Biomed. Meter. Res., 25(7)
F 889-902,1991D
12)GIOVANNA LEZZI ,SERGIO ORLANDI,GABRIELE PECOA, ADRIANO PIATTELLI.:
"Histologic and Histomorphometric Evaluation of The Bone Response Around a
Hydroxyapatite=Coated Implant Retrieved After 15 years" The International
Journal of Periodontics&Restorative Dentistry Volume 17,Number2,2009
13)FRAYSSINET P, HARDY D, HANKER JS, GIAMMARA BL. Natural history of bone
response to hydroxyapatite-coated hip prostheses implanted in humans.Cell
Mater 1995;5:125-138
14)BLOCK, MS., GARDINER, D., KENT J.,MISIEH D., et. al, :Hydroxyapatite-coated
Cylindrical Implants in the posterior, Mandible 10-year Observations ; Int.J.Oral
Maxillofac.Implants,11:626-633,1996.
15)NISHIGUCHI, S., NAKAMURA, T., KOBAYASHI, M., KIM, H., MIYAJI, F. and
KOKUBO, T. :The effect of heat treatment on bone-bonding ability of alkali-treated
titanium; Biomaterials 20:491-500 ,1999.
16)LINKOW LI: Statistical analyses of 173 patients, J Oral Implantol 4F540-562, 1974D
17jADELL R, LEKHOLM U, ROCKLER B, et al: A 15 year study of osseointegreted
implants in the treatment of the edentulous jaw,Int j Oral Maxillofac surg
10:387-416, 1981.
18)ADELL R, LEKHOLM U, ROCKLER B, et al: Margnal tissue reactions at
osseointegreted titanium fixturesi1jFa 3 year longitudinal prospective study , Int j
Oral Maxillofac surg 15:39-52, 1996.
19)TONETTI MS, SCHMID J: Patheogenesis of implant failures, Periodontology 2000
4:127-138,1994.
20)MISCH CARL E.: Dental Implant ProstheticsF72-82D MosbyCInc. 2005
21)FROST HMFMechanical adaptation :Frost's mechanostat theory. In Martin RB,
Burr DB, editors: structure, function, and adaptation compact bone, New
York,1989,Raven Press
22)WONEJAE YU, YOON-JE JANG, HEE-MOON KYUNG Combined Influence of
Implant Diameter and Alveolar Ridge Width on Crestal Bone Stress: A Quantitative
JOMI Volume 24,Number1,2009
Eiji Kato, at The Kato Dental Clinic and is a member of theImplant & Tissue-Engineering Dental Network (ITDN), Tokyo, Japan.
from KIT News 2010/01/21
Discussion on Surface Properties of Implant -Potential of HA-coating-
Published by Japan Medical Materials Corporation
